Your Cart is Empty


NeoPUTTY® is a premixed bioactive bioceramic root and pulp treatment material with superior handling properties, promoting hydroxyapatite formation to trigger the healing process.
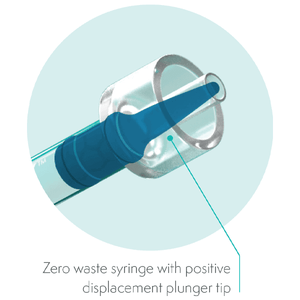
Its firm, non-tacky consistency, wash-out resistance and bioactivity makes it the preferred material for every pulp need. NeoPUTTY delivers a ready-to-use material for immediate placement with zero waste, saving cost and chair time.

Bioactive Bioceramic
No dry out
Highest radiopacity in its class
Resin-Free
Non-Staining
SKU ANPPK- 1.2 gm syringe – 16 doses2
SKU ANPSK – 0.65 gm syringe – 9 doses2
1NeoPUTTY is initially high in pH (alkaline/basic) when applied. Literature has shown such products to be antimicrobial in-vitro.
The anti-microbial effect against enterococcus faecalis and the compressive strength of two types of mineral trioxide aggregate mixed with sterile water or 2% chlorhexidine liquid. Holt DM, Watts JD, Beeson TJ, Kirkpatrick TC, Rutledge RE. J Endod. 2007 Jul;33(7):844-7.
2The # of doses varies depending on the treatment. A dose size of 0.075 gm was used here.